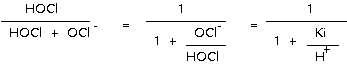|
|
|
|
|
When chlorine gas is dissolved in water, it hydrolyzes rapidly according to the following equation: Cl2 + H2O <---> HOCl + H+ + Cl- Complete hydrolysis occurs in a few tenths of a second at 18oC; at 0oC only a few seconds are needed. 2) Chemistry of Hypochlorous Acid The most important reaction in the chlorination of an aqueous solution is the formation of hypochlorous acid. This species of chlorine is the most germicidal of all chlorine compounds with the possible exception of chlorine dioxide. Hypochlorous acid is a weak acid which means that it tends to undergo partial dissociation as follows: HOCl <---> H+ + OCl- to produce a hydrogen ion and a hypochlorite ion. In waters of pH between 6.5 and 8.5 the reaction is incomplete and both species are present to some degree. The table for the percent undissociated HOCl species for the various temperature and pH values is shown below.
The percent OCl- ion is the difference between these numbers and 100. The percent distribution of the OCl- ion (hypochlorite ion) and undissociated hypochlorous acid (HOCl) can be calculated for various pH values as follows:
Where, Ki is a constant of HOCl ionization and is calculated from (H+) x (OCl-) / (HOCl). This constant is shown on below table.
At 20oC and pH 8, the percent distribution of HOCl is obtained from; 100 x [ 1 + (Ki / H+) ]-1 = 100 x [ 1 + (2.621 x 10-8 / 10-8) ]-1 = 100 / 3.61 = 27.65% HOCl is the most effective of all the chlorine residual fractions. This fraction is known officially in the industry as free available chlorine residual. Hypochlorous acid is similar in structure to water; hence, the formula HOCl is preferred to HClO. The germicidal efficiency of HOCl is due to the relative ease with which it can penetrate cell walls. This penetration is comparable to that of water, and can be attributed to both its modest size (low molecular weight) and to its electrical neutrality (absence of an electrical charge.) Other things being equal, the germicidal efficiency of a free available chlorine residual is a function of the pH, which establishes the amount of dissociation of HOCl to H+ and OCl- ions. Percent HOCl table shows the percentage of undissociated HOCl in a chlorine solution for various pH values an temperatures. Lowering the temperature of the reacting solution suppresses the dissociation; conversely, raising the temperature increase the amount of dissociation. The rate of dissociation of HOCl is so rapid that equilibrium between HOCl and OCl- ion is maintained, even though the HOCl is being continuously used. For example. if water containing 1 mg/l of titable free available chlorine residual has been dosed with a reducing agent that consumes 50 percent of the hypochlorous acid, the remaining residual will redistribute itself between HOCl and OCl- ion according to the values shown the percent HOCl Table. This is commonly referred to as the "reservoir" effect. 3) Hypochlorite Ion The OCl- ion, which is a result of the dissociation phenomenon, is a relatively poor disinfectant, because of its inability to diffuse through the cell wall of microorganisms. The obstacle to this passage is the negative electrical charge, as sub-staintiated to some extent by the fact that the activation energy for disinfect ion by HOCl is in the range of those for diffusion (E = 7,000 calories), whereas that of the OCl- ion is more characteristic of a chemical reaction (E = 15,000 calories). It is well known that the disinfecting efficiency of free available chlorine residual decreases significantly as the pH rises. At a pH above 9 there is little disinfecting power. At this pH level and at 20oC, 96 percent of the titrable free available chlorine will consist of the OCl- ion. This is an indication of the low germicidal efficiency of the OCl- ion. In general the relative efficiencies of the OCl- and HOCl for inactivation of cysts are summarized as follows:
4) Hypochlorite Solutions The exact same chemical reaction occurs when hypochlorite solutions are used instead of aqueous chlorine solutions. If, for example, common bleach (sodium hypochlorite) is used, it appear in water to form hypochlorous acid: NaOCl + H2O <---> HOCl + NaOH The hypochlorous acid formed by this reaction precedes to dissociate as per above reaction described in Chemistry of Hypochlorous Acid. 5) Chlorine and Nitrogenous Compounds The most important and undoubtedly the most complex chemistry of water chlorination is its reaction with various forms of nitrogen naturally occurring in water. If the water to be treated did not contain nitrogenuous compounds, the chlorination of water would be extremely simple. The total residual would always be free available chlorine. There would be no problem with quantitative determination of residuals. The disinfecting efficiency of chlorine could be predicted and controlled within a negligible margin of error. However, this is not the case. Nitrogen appears in most natural waters and in varying amounts as either organic or inorganic nitrogen. These compounds of nitrogen and their relationship to chlorination will be considered in the general grouping as follows:
The chemical state of any nitrogen compound found in nature is a function of time in the overall life process of all plants. The amounts of these various forms of nitrogen relate directly to the sanitary quality of the water to be treated. These compounds fit very definitely in time on the nitrogen cycle of nature's own processes of purification. The reaction of chlorine with any compound containing the nitrogen atom with one or more hydrogen atoms attached will form a compound broadly classified as an N-chloro compound, or, more commonly, as chloramine. There are two distinct classes of chloramines - organic and inorganic. The inorganic chloramines are formed by the reaction of chlorine in an aqueous solution with free ammonia naturally occurring in the water being treated. These chloramines are relatively simple compounds. To be continued. Please press the next button.... |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||